Explain Why Atoms Have Different Isotopes
The example of two Isotopes and Isobars is iron and nickel. Stable isotopes either never decay or else decay very slowly.
5 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts
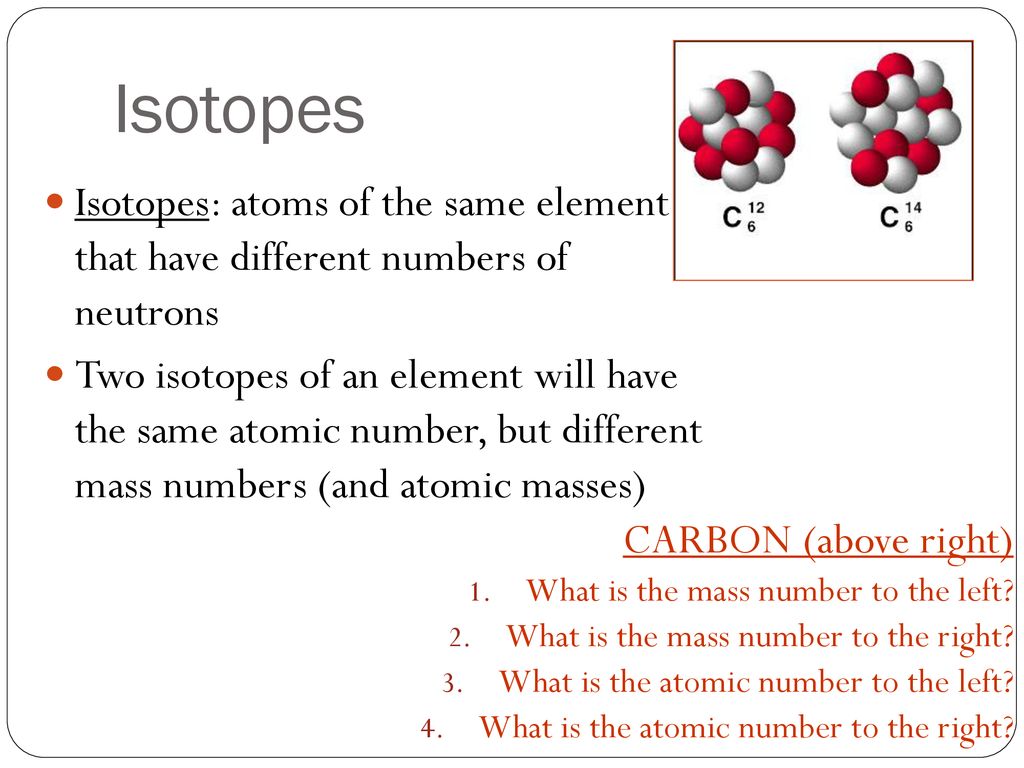
For example 12 C and 13 C are the most abundant isotopes in nature but 14 C also exists.

. The nuclei of isotopes contain different numbers of _____. Relative abundance is the amount of each isotope as the percentage for that element occurring on the Earth All Carbon isotopes have the same atomic number. Some isotopes are unstable and will undergo radioactive decay to become other elements.
Carbon-14 14 C is unstable and only occurs in trace amounts. There are three isotopes of carbon found in nature carbon-12. Of the same element must have the same number of protons but they can have different.
Naturally occurring samples of most elements are mixtures of isotopes. Atoms of elements with different numbers of neutrons are called isotopes of. Carbon-12 12 C is the most abundant of the carbon isotopes accounting for 9889 of carbon on Earth.
They do however tend to have the same chemical properties. For example carbon has six protons and is atomic number 6. Atoms in a chemical element that have different numbers of neutrons than protons and electrons are called isotopes.
In a chemical laboratory isotopes of an element appear and react the same. An elements atoms always have the same number of protons. Elements have families as well known as isotopes.
The three isotopes of carbon can be referred to as carbon-12 C 6 12 carbon-13 C 6 13 and carbon-14 C 6 14. The number of protons for different isotopes of an element does not change. The presence of more or less number of neutrons makes the atom unstable and so these isotopes are found in low amounts in nature.
Isotopes are variants of the same element that have different numbers of neutrons and thus potentially different physical properties. This difference in neutron amount affects the atomic mass A but not the atomic number Z. Some atoms gain or lose a neutron.
Write the formula for calculating Average Atomic Mass. Explain why these atoms have the same atomic number. Hydrogen is a common element on earth.
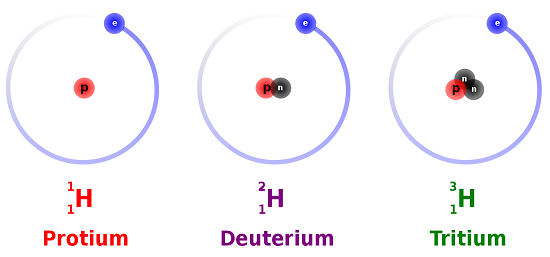
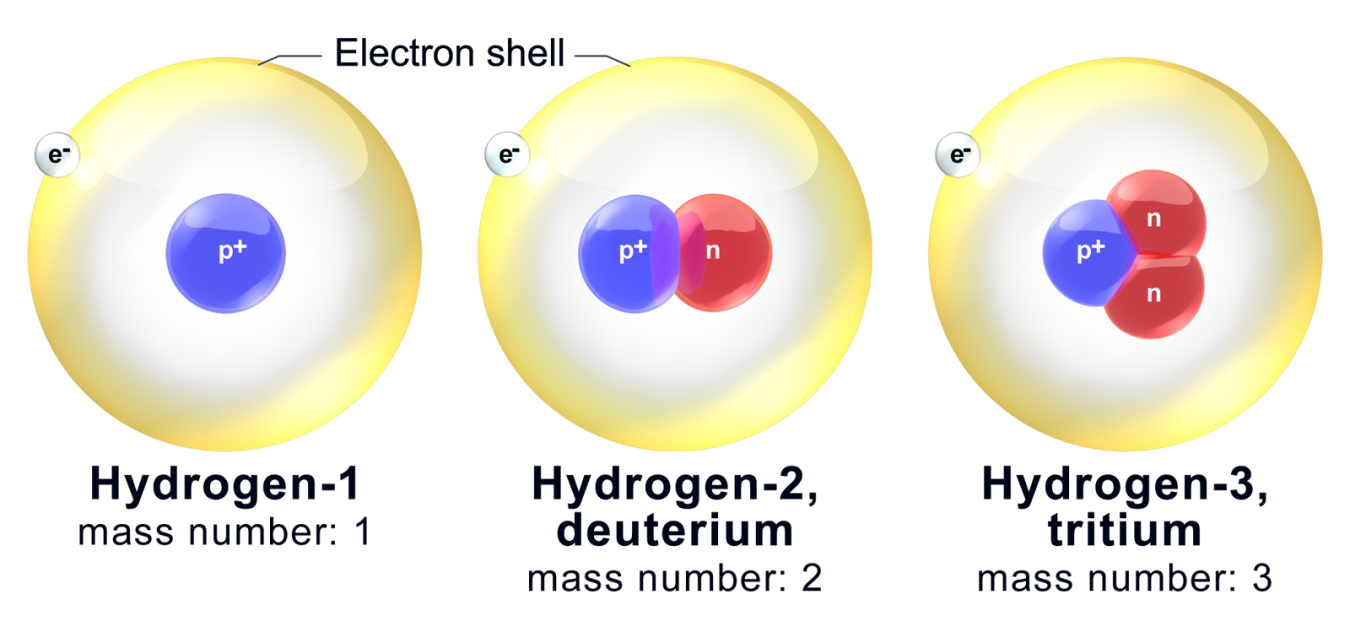
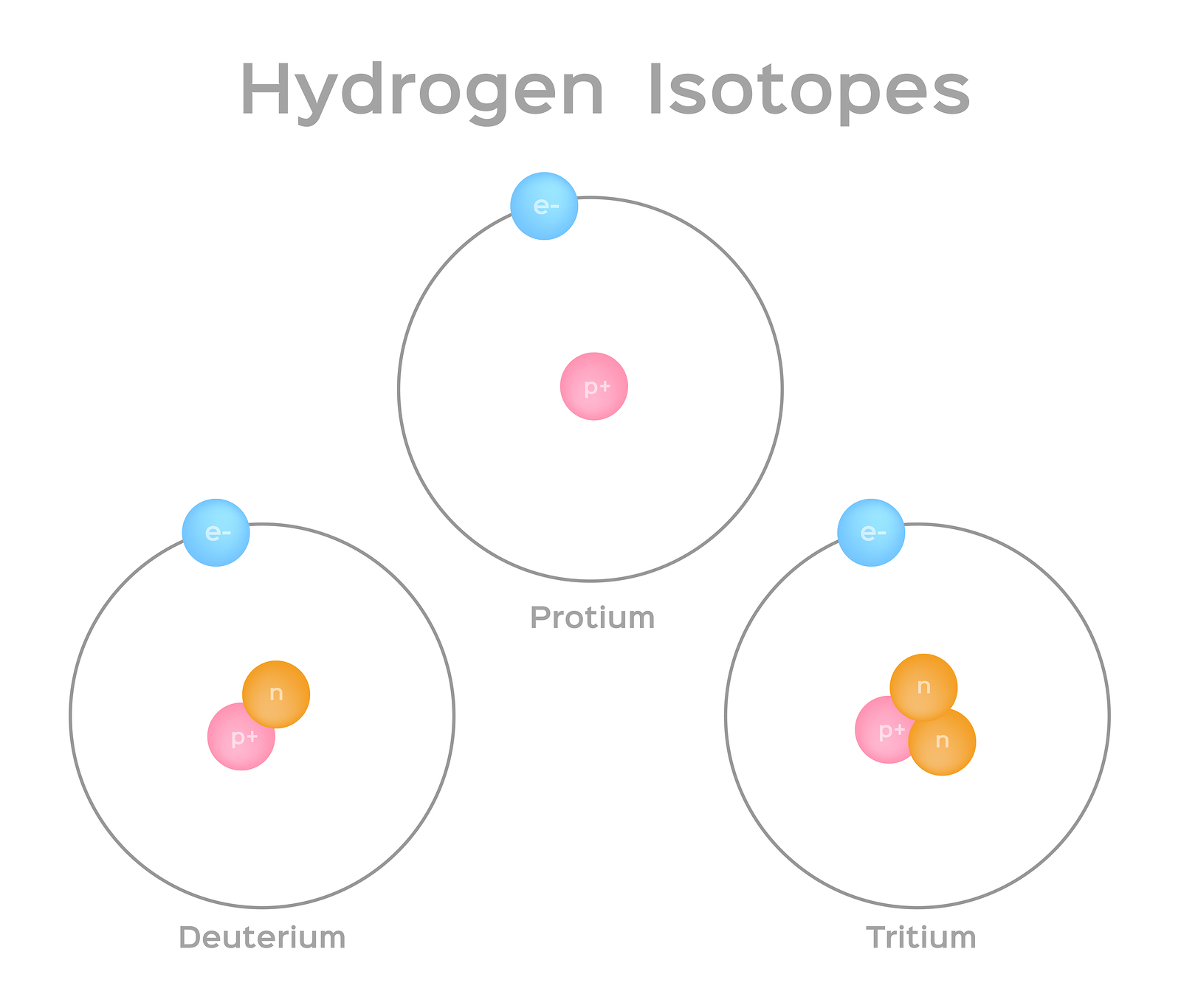
Hydrogens atomic number is 1 its nucleus contains 1 proton. Isotopes are atoms of the same element that contain different numbers of neutrons. The atoms in a particular element have an identical number of protons and electrons but can have varying numbers of neutrons.
Isotopes are samples of an element with different numbers of neutrons in their atoms. Most elements exist in nature as multiple types of isotopes. Now each isotope is named on the basis of its mass number which is the total combined number of neutrons and protons in an atom.
Since the atomic number is equal to the number of protons and the atomic mass is the sum of protons. The _____ _____ of an isotope is the sum of the number of protons and neutrons in its nucleus. Typically an atom has the same number of protons and neutrons.
Not all isotopes are radioactive. Unstable isotopes most commonly emit alpha particles He 2 and electrons. 17 protons 17 electrons 35 - 17 18 neutrons Isotopes are atoms of an element with the normal number of.
This gives rise to different mass numbers. Changing the number of neutrons in an atom does not change the element. Isotopes are atoms with the same number of protons but that have a different number of neutrons.
Mass number determines the physical properties while atomic number determines the chemical properties. The reason for this is because isotopes of an element have the same number of electrons as an atom of that element but they have different number of neutrons which affects the mass number. It has the same atomic mass but different atomic no.
Lets use carbon as an example. Isotopes are atoms of the same element that contain an identical number of protons but a different number of neutrons. Isotopes of an atom have different atomic masses and exhibit different properties but they are still the same element.
When the number of neutrons in an atom changes an isotope is formed. Because there are different ways of stabilizing the protons there are different isotopes. For these species the number of electrons and protons remain constant.
Radioactive isotopes undergo decay. Isotopes of an element share the same number of protons but have different numbers of neutrons. Isotopes are atoms that have the same number of protons and electrons but a different number of neutrons.
Atoms of the same element always have the same number of protons. All the atoms of an element have the same number of protons atomic. Calcium-42 calcium-48 calcium-43 and calcium-46 atoms.
Neutrons protons and positrons can also be emitted and electrons can be captured to attain a. 2 mass of isotope x relative abundance Neutrons exist to stabilize the nucleus without them the nucleus would consist of nothing but positively-charged protons in close proximity to one another. The number of protons in a nucleus determines the elements atomic number on the Periodic Table.
Despite having different numbers of neutrons isotopes of the same element have very similar physical properties. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons. Isotopes do differ in their stability.
Isotopes are atoms of the same element which differs in the number of neutrons present inside the nucleus. Isotopes are atoms of the same element but with different number of neutrons. Isotopes are atoms that have the same atomic number but different mass numbers due to a change in the number of neutrons.
When an isotope decays the starting material is the parent isotope. Their chemical property is different because there is a difference in the number of electrons. This is because an additional number of neutrons compensates the difference in the number of nucleons.
This means that each of these atoms has.
Doe Explains Isotopes Department Of Energy
Isotope Basics Nidc National Isotope Development Center
Isotopes And Ions Do Now Explain How Atoms Of Different Elements Differ From One Another Give A Specific Example Ppt Download
No comments for "Explain Why Atoms Have Different Isotopes"
Post a Comment